Urgency for Enhanced Policy Intervention to Combat Avian Influenza in Dairy Cows
Introduction
Avian influenza, traditionally known in its highly pathogenic forms among bird populations, has recently made alarming advances into new territories—including dairy cows. This zoonotic virus poses a threat to animal health and potentially to humans as well, linking public health and agricultural concerns. The revelation that dairy cows can contract and spread H5N1 has broadened the scope of this disease’s impact, raising fears of another possible pandemic if the virus adapts to transmit between humans more efficiently (Centers for Disease Control and Prevention, 2024a). The dairy industry is highly relevant for agricultural sectors worldwide, contributing significantly to national economies and supporting millions of livelihoods. The spread of avian influenza in dairy cows across the United States, as shown in Figure 1, is a major concern for the dairy industry. This is because dairy products generate about 3.2 million American jobs with a direct economic contribution of more than $250 billion (International Dairy Foods Association, 2024).

The introduction of a virulent disease such as avian influenza into this critical sector could have devastating economic repercussions, reminiscent of the foot-and-mouth disease outbreak in the early 2000s (Enserink, 2001). The emergence of avian influenza in dairy cows highlights the ongoing challenges posed by zoonotic diseases, which have repeatedly crossed species barriers to affect humans—most notably seen during the COVID-19 pandemic. The ability of these pathogens to exploit interspecies interactions highlights a pressing need for global awareness and coordinated response strategies. Therefore, addressing the spread of avian influenza within dairy populations is not just a matter of animal health but also crucial for safeguarding global public health and economic stability (Nowogrodzki, 2024).
Background
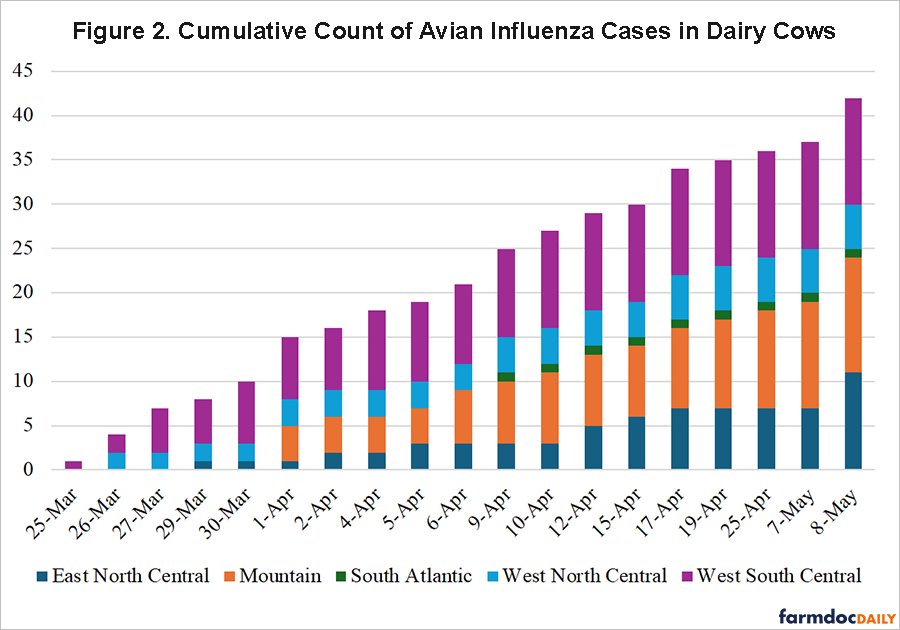
Avian influenza, or bird flu, is caused by influenza type A viruses which affect several species of birds, often leading to severe epidemics. While typically confined to avian species, certain strains like H5N1 have an alarming capacity to infect mammals, including humans, and result in a high fatality rate (Abdelkawi et al., 2024). In the case of dairy cows, recent incidents in the United States have highlighted a new vector for zoonotic transmission, worsening concerns about the virus’s adaptability and mutability. Transmission to cows can occur through direct contact with infected birds or contaminated environments, emphasizing the importance of stringent biosecurity measures. The recent outbreaks have underscored the unpredictability of avian influenza. Since the initial detection in a Texas dairy herd on March 25, 2024, avian influenza has been identified on 42 premises handling dairy cows across nine U.S. states, as shown by the cumulative count by Census region in Figure 2. The potential economic repercussions are profound. Infected farms may encounter the culling of affected herds and face significant disruptions in milk production. For instance, during the 2024 outbreaks, affected regions reported a sharp decline in milk yield, with corresponding losses in farm revenue (Burrough et al., 2024).
Compounding these challenges is the discovery that cows possess receptors similar to those found in humans, specifically receptors that facilitate influenza virus entry into cells (Kristensen et al., 2024). This similarity increases the likelihood that virus variants adapted to infect cows could more readily jump to humans, which is a scenario that could mirror past influenza pandemics. The biological bridge marks a critical point of concern for public health officials, heightening the urgency for effective monitoring and response strategies to curb the spread of the virus and prevent a potential public health crisis (European Food Safety Authority et al., 2024). The intersection of these factors—viral adaptability, potential economic impact, and biological predisposition—makes the avian influenza outbreaks in dairy cows a significant concern for global health.
Current Policy Response
In response to the avian influenza outbreaks among dairy cattle, U.S. governmental agencies, including the U.S. Department of Agriculture (USDA) and the U.S. Department of Health and Human Services (HHS), have implemented a series of coordinated actions to control the spread of the virus and mitigate its impact. These measures include stringent movement restrictions on affected livestock, comprehensive testing protocols across farms, and substantial financial assistance worth about $200 million to impacted producers. The USDA has enforced a Federal Order to restrict the movement of lactating dairy cattle from regions identified with H5N1 outbreaks (U.S. Department of Agriculture, 2024). This move aims to prevent the spread of avian influenza to uninfected areas and should help to manage the risk of potential transmission to humans. Moreover, the agency has ramped up its testing efforts, mandating regular screenings for H5N1 in both symptomatic and asymptomatic cattle within designated surveillance zones.
Financially, in collaboration with the HHS, the USDA has extended significant support to affected dairy producers. This includes compensations for lost milk production under the Emergency Assistance for Livestock, Honey Bees, and Farm-raised Fish Program (ELAP) and subsidies for implementing enhanced biosecurity measures (U.S. Department of Agriculture, 2024). Secretary Tom Vilsack states, “We are now moving into a phase of equipping producers to reduce the risk.” Vilsack also said the tools offered by the department represent a value of up to $28,000 per premises to support increased biosecurity activities over the coming 120 days. Table 1 summarizes the measures that are implemented by the USDA and HHS. However, the effectiveness of these policy measures has been met with mixed reviews. While some industry experts commend the government’s quick response, others critique the practical implementation of biosecurity and testing measures (Bartels, 2024). Dr. Meghan Davis, an epidemiologist at the Johns Hopkins Bloomberg School of Public Health and a former dairy veterinarian, notes, “I think that we are a bit behind on the surveillance…” and “that biosecurity is the only thing that we can do right now, at least until we have more answers in terms of the dynamic of the virus.” This sentiment is echoed by some producers who find the guidelines challenging to implement due to their complexity and the costs involved.
| Table 1. USDA Assistance and Financial Support for H5N1 Affected Dairy Producers | ||
| Assistance Category | Description | Financial Support |
| Biosecurity Improvement | USDA aids with on-site biosecurity enhancements to reduce H5N1 spread. | – |
| Financial Tools | Assistance for lost milk production due to H5N1. | – |
| Pre-movement Testing | Builds on Federal Order for testing before moving cattle. | – |
| Human and Animal Spread Prevention | Financial support for producers providing PPE and laundering services. | Up to $2,000 per affected premises per month |
| Study Participation Incentives | Financial incentives for workers participating in USDA/CDC studies. | Variable incentives |
| Biosecurity Planning Support | Assistance to develop biosecurity plans; $100 payment for using an in-line sampler. | Up to $1,500 per premises; $100 for sampler purchase |
| Milk Disposal | Funding for heat treatment of milk to inactivate the virus. | Up to $2,000 per affected premises per month |
| Veterinary Cost Reimbursement | Covers costs for veterinary care and testing of cattle with H5N1. | Up to $10,000 per affected premises |
| Shipping Costs for Testing | Covers shipping samples to NAHLN labs for influenza A testing. | Up to $100 per premises per month (2 shipments) |
| Total Support Value | Comprehensive support for biosecurity activities. | Up to $28,000 per premises over 120 days |
| Note: Data comes from the U.S. Department of Health and Human Health Services (2024). | ||
Additionally, the geographical spread of the virus despite the restrictions raises questions about the sufficiency and enforcement of movement controls. The Centers for Disease Control and Prevention (CDC) reports that, although the measures have localized the outbreaks to some extent, sporadic cases outside the controlled zones suggest that more stringent enforcement might be necessary (Centers for Disease Control and Prevention, 2024b). In sum, while the U.S. government’s response to the avian influenza outbreak in dairy cows has been proactive and substantial in financial support, the effectiveness of containment and biosecurity measures remains under scrutiny. Continued evaluation and possibly enhanced strategies are crucial to address the gaps identified by agricultural stakeholders and experts in the field. The situation underscores the challenges of managing zoonotic diseases within the agricultural sector and the need for adaptive responses as new information emerges.
Policy Gaps
While the policy responses from USDA and HHS to the avian influenza outbreak in dairy cows have been robust, several gaps persist that may undermine the effectiveness of these measures. Key areas of concern include delays in disease diagnosis, inconsistencies in biosecurity practices, and potential lapses in inter-agency coordination. One significant gap is the speed of diagnosis. Rapid and accurate detection of avian influenza is crucial for containing outbreaks before they spread. However, the current diagnostic infrastructure is likely insufficient to handle the required tests during a widespread outbreak (European Food Safety Authority et al., 2024). Delays in confirming cases can lead to missed opportunities for early containment, allowing the virus to spread further undetected. For example, in some instances, test results for avian influenza in poultry have taken up to a week, during which time the virus could infect an entire dairy cow herd (McCafferty, 2024).
Biosecurity measures, although well-intended, also show inconsistencies in implementation. Effective biosecurity is critical to prevent the introduction and spread of the virus on farms. Yet, adherence can vary significantly among producers, often due to the high costs and logistical complexities of implementing these measures (Merrill et al., 2019). This variability can create vulnerabilities where the virus can enter and spread within and between herds. Inter-agency coordination between the USDA, the HHS, and local health departments is crucial for a unified response to this zoonotic threat. However, discrepancies in protocols and communication can lead to fragmented responses, reducing the overall effectiveness of the interventions. For instance, some states may apply federal guidelines differently, leading to uneven enforcement and protection levels across the United States.
The economic and health implications of these policy gaps are significant. Economically, delays in diagnosis and variable biosecurity can lead to greater spread of the disease, increasing the cost of containment and mitigation, leading to higher economic losses for farmers from potential culls and decreased milk yields. From a public health perspective, ineffective containment and biosecurity can increase the risk of zoonotic transmission, posing health risks to farm workers and the wider community if the virus adapts to more efficient human-to-human transmission (Ly, 2024). Such a scenario would be devasting considering the 56% fatality rate reported for avian influenza in humans (World Health Organization, 2024). Addressing these gaps requires increased funding and a focus on enhancing diagnostic capacities, standardizing and enforcing biosecurity measures, and improving the coordination between all agencies involved in disease management. It involves overcoming coordination barriers between federal and state agencies and across different agencies. Such steps are crucial to safeguard both the agricultural economy and public health.
Potential Ways Forward
To address the current gaps in the response to avian influenza outbreaks in dairy cows, a comprehensive strategy that encompasses improved funding, surveillance, international collaboration, and innovative research is required. Strengthening these priority areas could significantly enhance the ability to effectively manage and control avian influenza outbreaks, reducing potential economic impacts and health risks.
Immediate increases in funding for and sustained investment in biosecurity measures are critical. This funding should support implementing advanced surveillance systems that detect the virus early, particularly in remote and rural areas where veterinary services might be less accessible (Worsley-Tonks et al., 2022). Enhanced surveillance could include digital tracking and AI-driven predictive analytics to monitor herd health and predict outbreak patterns. This would involve advancing private-public partnerships and data-sharing protocols. Additionally, more resources should be allocated to train personnel on the ground, ensuring they are well-equipped to handle outbreaks swiftly (Kapoor and Dhama, 2014). Because avian influenza does not respect borders, ensuring international cooperation is vital, particularly between Canada, Mexico, and the United States. A global network for sharing real-time data on avian influenza outbreaks can enable countries to preemptively tighten biosecurity measures and prepare responses based on the activities in neighboring regions and beyond. Furthermore, international human and animal health bodies like the World Health Organization and the Food and Agriculture Organization should work closely with national governments to standardize response strategies and protocols, ensuring a cohesive global response to avian influenza outbreaks.
Investing in genetic research to explore the resistance of cows to avian influenza could pave the way for breeding programs that prioritize genetic resistance to the virus (Idoko-Akoh et al., 2023). Additionally, funding should be directed toward developing new vaccines designed to combat the strains of influenza found in cows. Such vaccines could lessen the severity of outbreaks and reduce the spread of avian influenza among cattle herds. Forging stronger partnerships between government agencies and private sector stakeholders is essential to achieve this goal. These collaborations can bring about innovations in vaccine development and biosecurity technologies more rapidly than public sector efforts alone. Private sector involvement can also provide the necessary capital and logistics expertise to scale up biosecurity measures effectively. For instance, technology companies can partner with agricultural businesses to deploy IoT sensors for real-time livestock health monitoring, enhancing disease detection capabilities and reducing the risks associated with avian influenza spread.
Finally, there should be a concerted effort to enhance awareness and education among farmers and the dairy industry about the importance of biosecurity and early detection (Denis-Robichaud et al., 2019). Tailored educational programs and extension activities can inform stakeholders about best practices and the latest disease management technologies, fostering a culture of awareness and proactive health management within rural communities. By addressing these key areas, the policy response to avian influenza in dairy cows can become more robust, adaptive, and effective, helping to safeguard the agricultural sector and public health from future threats from avian influenza in dairy herds.
Conclusion
The avian influenza outbreak in dairy cows has revealed a critical vulnerability at the intersection of animal health and public safety. Effective management of this zoonotic threat demands immediate and comprehensive agricultural and public health policy measures, enhanced surveillance, and stronger biosecurity practices. It is imperative that federal and state agencies adopt a robust approach to prevent the spread of this virus, which has already shown its potential to disrupt both the dairy industry and the broader agricultural ecosystem. Policymakers, industry stakeholders, and the scientific community must collaborate closely to strengthen defenses against avian influenza. This includes substantial and sustained investment in research for developing resistant cattle breeds, more effective vaccines, and implementing cutting-edge surveillance technologies to predict and prevent outbreaks before they escalate. The ongoing situation with avian influenza in dairy cows is a stark reminder of the broader implications for global health security. Proactive and aggressive measures are not just about protecting livestock—they are critical to preventing potential future pandemics that could arise from zoonotic diseases. As such, the effort to tackle avian influenza is a matter of major importance for the agricultural sector and a paramount public health objective, essential for safeguarding human populations worldwide against emerging infectious diseases.
References
Abdelkawi, Abdullah, Zaineb Zinoune, Aliyah Slim, and Yashwant V. Pathak. 2024 "Avian Influenza Outbreaks over the Last Decade: An Analytical Review and Containment Strategies." Rising Contagious Diseases: Basics, Management, and Treatments: 42-49. https://doi.org/10.1002/9781394188741.ch5.
Bartels, Meghan. 2024. "How Bird Flu Caught the Dairy Industry Off Guard." Scientific American, May. https://www.scientificamerican.com/article/how-bird-flu-caught-the-dairy-industry-off-guard/.
Burrough, Eric R., Drew R. Magstadt, Barbara Petersen, Simon J. Timmermans, Phillip C. Gauger, Jianqiang Zhang, Chris Siepker et al. 2024. "Highly pathogenic avian influenza A(H5N1) clade 2.3.4.4b virus infection in domestic dairy cattle and cats, United States, 2024." Emerging Infectious Diseases. https://doi.org/10.3201/eid3007.240508.
Centers for Disease Control and Prevention. 2024a. "Current H5N1 Bird Flu Situation in Dairy Cows." Accessed May 13, 2024. https://www.cdc.gov/flu/avianflu/mammals.htm.
Centers for Disease Control and Prevention. 2024b. "Current U.S. Bird Flu Situation in Humans. " Accessed May 13, 2024. https://www.cdc.gov/flu/avianflu/inhumans.htm.
Denis-Robichaud, J., D. F. Kelton, C. A. Bauman, H. W. Barkema, G. P. Keefe, and J. Dubuc. 2019. "Canadian dairy farmers' perception of the efficacy of biosecurity practices." Journal of Dairy Science 102, no. 11: 10657-10669. https://doi.org/10.3168/jds.2019-16312.
Enserink, Martin. 2021. "Barricading U.S. Borders Against a Devastating Disease." Science 291, no. 5512: 2298-2300. https://www.science.org/doi/10.1126/science.291.5512.2298.
European Food Safety Authority, European Centre for Disease Prevention and Control. Angeliki Melidou, Theresa Enkirch, Katriina Willgert, Cornelia Adlhoch et al. 2024. "Drivers for a pandemic due to avian influenza and options for One Health mitigation measures." EFSA Journal, 22(4), p.e8735. https://doi.org/10.2903/j.efsa.2024.8735.
Idoko-Akoh, Alewo, Daniel H. Goldhill, Carol M. Sheppard, Dagmara Bialy, Jessica L. Quantrill, Ksenia Sukhova, Jonathan C. Brown et al. 2023. "Creating resistance to avian influenza infection through genome editing of the ANP32 gene family." Nature Communications 14, no. 1: 6136. https://doi.org/10.1038/s41467-023-41476-3.
International Dairy Food Association. 2024. "The Economic Impact of Dairy Products." Accessed May 13, 2024. https://www.idfa.org/dairydelivers.
Kapoor, Sanjay, and Kuldeep Dhama. 2014. "Prevention and Control of Influenza Viruses." In: Insight into Influenza Viruses of Animals and Humans: 163-216. Springer, Cham. https://doi.org/10.1007/978-3-319-05512-1_11.
Kristensen, Charlotte, Henrik Elvang Jensen, Ramona Trebbien, Richard J. Webby, and Lars Erik Larsen. 2024. "The avian and human influenza A virus receptors sialic acid (SA)-α2, 3 and SA-α2, 6 are widely expressed in the bovine mammary gland." bioRxiv: 2024-05. https://doi.org/10.1101/2024.05.03.592326.
Ly, Hinh. 2024. "Highly pathogenic avian influenza H5N1 virus infections of dairy cattle and livestock handlers in the United States of America." Virulence 15, no. 1: 2343931. https://doi.org/10.1080/21505594.2024.2343931.
McCafferty, Caitlin, ed. 2024. "New avian influenza test could help protect poultry and public health." January 9. Accessed May 13, 2024. https://www.dvm360.com/view/groundbreaking-avian-influenza-test-could-protect-poultry-and-public-health.
Merrill, Scott C., Susan Moegenburg, Christopher J. Koliba, Asim Zia, Luke Trinity, Eric Clark, Gabriela Bucini et al. 2019. "Willingness to comply with biosecurity in livestock facilities: evidence from experimental simulations." Frontiers in Veterinary Science 6: 156. https://doi.org/10.3389/fvets.2019.00156.
Nowogrodzki, Julian. 2024. "Bird flu in US cows: is the milk supply safe?" News Explainer, April 25. Nature. https://doi.org/10.1038/d41586-024-01221-2.
U.S. Department of Agriculture, Animal and Plant Health Inspection Service. 2024. "Highly Pathogenic Avian Influenza (HPAI) Detections in Livestock." Accessed May 13, 2024. https://www.aphis.usda.gov/livestock-poultry-disease/avian/avian-influenza/hpai-detections/livestock.
U.S. Department of Health and Human Health Services. 2024. "Fact Sheet: USDA, HHS Announce New Actions to Reduce Impact and Spread of H5N1." Accessed May 13, 2024. https://www.hhs.gov/about/news/2024/05/10/fact-sheet-usda-hhs-announce-new-actions-reduce-impact-spread-h5n1.html.
World Health Organization, 2024. "Avian Influenza Weekly Update Number 945." Accessed May 13, 2024. https://iris.who.int/handle/10665/375483
Worsley-Tonks, Katherine EL, Jeff B. Bender, Sharon L. Deem, Adam W. Ferguson, Eric M. Fèvre, Dino J. Martins, Dishon M. Muloi et al. 2022. "Strengthening global health security by improving disease surveillance in remote rural areas of low-income and middle-income countries." The Lancet Global Health 10, no. 4: e579-e584. https://doi.org/10.1016/S2214-109X(22)00031-6.
Disclaimer: We request all readers, electronic media and others follow our citation guidelines when re-posting articles from farmdoc daily. Guidelines are available here. The farmdoc daily website falls under University of Illinois copyright and intellectual property rights. For a detailed statement, please see the University of Illinois Copyright Information and Policies here.







